Outer energy level is the outermost energy level of the atom. Atoms of group 18 elements have eight valence electrons or two in the case of helium.
Valence electrons can greatly impact the properties of atoms of the same element.
. This process is illustrated below for the elements fluorine oxygen and nitrogen. Neon has a complete outer 2n shell containing eight electrons. Neon has a complete outer 2n shell containing eight electrons.
Valence electrons are electrons in the last shell of an atom. To start off the electrons on the outermost energy level of an atom are called valence electrons. Atoms of metals tend to lose all of their valence electrons which leaves them with an octet from the next lowest principal energy level.
March 31 2014 January 23 2021 Surfguppy 1 Comment Energy. They do not participate in any bond formation or chemical reaction because they are stable. In fact over 3500 isotopes are known for the different elements.
Any compound that increases the number of hydroxide ions when dissolved in water and whose solution tastes bitter feels slippery and can change the color of certain compounds. When an atom gains or loses electrons it has an electrical charge. These elements already have a full outer energy level so they are very stable.
Ask students to compare the number of electrons in the outermost energy level for the atoms in a group. These numbers are quantum numbers. Make a table of the three principal subatomic particles that make up the atom comparing their relative mass charge and location within the atom.
The hydrogen ion bonded to a molecule of water H 3 O the form in which hydrogen ions are found in aqueous solution. Thus by losing the lone electron in its third energy level sodium achieves stability and becomes a cation NaOn the other hand chlorine atomic number 17 needs only one electron to fill its valence shell. Most elements exist as isotopes.
An atom is stable when its outer energy level is filled with electrons. In contrast chlorine and sodium have seven and one in their outer shells respectively but theoretically they. In contrast chlorine and sodium have seven and one in their outer shells respectively but theoretically they.
Atoms are always attracted to other atoms. The atomic number is a unique element identifier and is an indicator of the. Atoms form compounds in ways that give them eight valence electrons.
Elements in other groups vary in their reactivity but are generally less reactive than elements in groups 1216 or 17 Valence Electrons and Electricity 1. Definition Obits and Energy Level. When atoms collide with other atoms they bond automatically.
All of the elements in Group 1 of the periodic table as we have demonstrated have the same number of electrons in their outermost energy level and the sublevel of that outermost energy level is also the same for all Group 1 elements. The electrons of the outermost energy level determine the energetic stability of the atom and its tendency to form chemical bonds with other atoms to form molecules. All the energy levels are denoted by integers eg.
The first energy level K contains 2 electrons second energy level 8 third energy level 18 and fourth energy level has 32. A molecule is produced. For instance hydrogen lithium sodium and potassium all have 1 electron on their outer energy level.
By accepting an electron chlo-rine achieves stability and becomes an anion. Outermost energy level has a great effect on how that atom can react with other atoms. The range of the quantum number can fluctuate between the lowest energy level to the highest energy level.
Atoms are at their most stable when their outermost energy level is either. Whatever is the most important use of an elements atomic What else can we know from a neutral atoms atomic number. Valence electrons are electrons in the highest principal energy level.
The electron is one of the most important factors in determining how an atom will react with another atom or. It turns red litmus paper blue. H2 and O2 share electrons to become h2o but the outermost level is not complete and find homework help for other Science questions at eNotes.
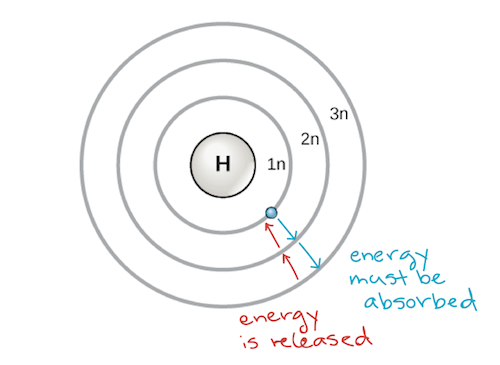
Most atoms are unstable unless they are combined with other atoms. Atoms in this condition are chemically active and will tend to gain lose or share electrons in order to complete their outermost energy levels. This is a very unstable arrangement and hydrogen gas undergoes a variety of reactions so as to reach a stable electron configuration where its energy level is either empty of electrons or filled with electrons.
The valence shell outermost energy level containing electrons and is full. Atoms are made of protons and neutrons making up the nucleus and electrons orbiting in energy shells around the nucleus. Most atoms are less stable when they combine with other atoms.
These atoms are known as inert atoms. Atoms of nonmetals tend to gain electrons in order to fill their outermost principal energy level with an octet. The atomic mass A of an element is the sum of the protons and neutrons within that element.
When atoms share electrons covalent bonds are formed between the atoms. It is known as. The electrons of the outermost energy level determine the energetic stability of the atom and its tendency to form chemical bonds with other atoms to form molecules.
While smaller atoms may or may not have completely filled orbitals the inner orbitals 1s 2s and 3p orbitals are fully engaged in all. Up to 24 cash back More commonly the outermost electron level is not completely filled. Students should realize that each atom in a group has the same number of electrons in its outermost energy level.
When nonmetal atoms gain electrons they often do so until their outermost principal energy level achieves an octet. You can tell how many valence electrons an. Hydrogen only has one electron in its lowest energy level.
The atoms that have filled the outermost shell with 8 atoms do not participate in any chemical reaction or they do not even bond with other atoms to form bond. Atoms of the same element have the same atomic number that have different numbers of neutrons are called isotopes. The valence electrons are the electrons in the outermost shell or last energy level of an atom.
N1 or 2 3 and so on. F e F 1 s 2 2 s 2 2 p 5 1 s 2 2 s 2 2 p 6 octet O 2 e O 2. Each energy shell has a maximum amount of electrons it can contain within the shell.
Chemical Bonding Biology For Non Majors I

Atomic Structure Of Matter Energy Levels Electronic Distribution Chemical Activity Science Online



0 Comments